 Patient Information NuvaRing® (NEW-vah-ring) (etonogestrel/ethinyl estradiol vaginal ring) Hormonal birth control methods help
Patient Information NuvaRing® (NEW-vah-ring) (etonogestrel/ethinyl estradiol vaginal ring) Hormonal birth control methods helpGlossary Study log administrators: see the information if you have registration or results. Search terms WarningManagement of Breakthrough Bleeding During Extended Therapy Use With NuvaRing® The safety and scientific validity of this study is the responsibility of the sponsor and researchers of the study. The inclusion of a study does not mean that it has been evaluated by the United States Federal Government. Read our details. ClinicalTrials.gov Identifier: NCT00475553 Status of recruitment : Completed First publication: 21 May 2007 Last Updated: December 18, 2009 State or disease Intervention/treatment Phase Bleeding Breakthrough Progress points Others: remove the ring if the bleeding or stain occurs more than 5 days Others: If the bleeding does not remove the ring Non-Applicable Hormonal contraception is undergoing a change in a 21/7-day regime in which a woman uses estrogen and progestin therapy combined for 21 days followed by 7 days of hormone-free interval (HFI). It is well documented that women can experience a greater incidence of mood changes, headaches and cramps that begin before and during this 7 days HFI. By reducing HFI and expanding active combined birth control therapy, women are expected to experience greater satisfaction with their birth control regime and will experience fewer negative side effects associated with an HFI. The most common reason for the interruption of an extended contraception regime is irregular bleeding. The objective of this research study is to evaluate the best way to manage this discovery and bleeding. The ease of use and acceptability of a flexible NuvaRing regime will also be evaluated. A comparison of the symptoms of cyclic humor, pelvic pain and headaches will take place between a standard regimen 21/7 and an extended regimen. The birth control ring used in this study contains both a estrogen (stradiol ethyl) and a progestin (etonogestrel). These are synthetic hormones (made by man). The amount of ethinyl is released in the bloodstream every day is 120mcg and the amount of etonógitos is 150mcg. The NuvaRing®, by Organon, is approved by the FDA for contraceptives, but is not approved for use in an extended regime. Therefore, its use in this study is considered investigative. Design table for study information Type of study: Intervention (Clinical judgment) Actual Registration : 75 participants Allocation: Random Model of intervention: Side allocation Masking: None (Open Label) Primary objective: Treatment Official title: The frequency and management of the elimination of progress during extended therapy with the transvaginal birth control ring Start date of the study: May 2006 Actual primary completion date: May 2008 Actual completion of the study Date: May 2008 Gun Intervention/treatment Group 2 The subject will use the numbness and if they developed bleeding or stained for more than 5 days on the 6th day the ring would be removed and leave it out for 3 full days and reinsert the same ring the next day. All subjects would be filling a daily journal or calendar that would qualify their blood flow, pelvic pain, headaches, moods, how many pain pills were taken and how many pills, coatings or tampons would be used. Others: remove the ring if the bleeding or stain occurs more than 5 days Both groups of women will use the numbness continuously, that is, if the ring is placed on January 3, the ring would be removed and another inserted. March 3 would happen the same and so on. Group 2 if the bleeding or staining occurs for more than 5 days, then the sixth day the ring is removed and will keep it out for three more days and restart the same ring on the fourth day. Other name: etonogestrel/ethinyl estradiol Group 1 The subject is using the nuvaring continuously and would change monthly. If you develop high-range bleeding or staining, do not remove the ring until it is your time to change it. All subjects would be filling a daily journal or calendar that would qualify their blood flow, pelvic pain, headaches, moods, how many pain pills were taken and how many pills, coatings or tampons would be used. Others: If the bleeding does not remove the ring The subject is using nuvaring continuously. For example, she puts the ring on January 3, then February 3 is when that ring is removed and another is inserted. March 3 is the same. Other names: Choosing to participate in a study is an important personal decision. Talk to your doctor and family or friends about deciding to join a study. For more information on this study, you or your doctor may contact the study research staff using the contacts provided below. For general information, Design table for eligibility information Eligible ages for study: 18 to 45 years Eligible sexes for the study: Women Accept healthy volunteers: Yeah. Inclusion criteria: Exclusion criteria: In addition, NuvaRing® should not be used in women who currently have the following conditions: For more information on this study, you or your doctor may contact the study research staff using the contact information provided by the sponsor. Please refer to this study by your ClinicalTrials.gov identifier: NCT00475553 Design table for location information United States of America, Texas Scott White Hospital and Clinic Temple, Texas, United States, 76508 Design table for research information Principal investigator: Patricia Sulak, MD Scott and White & Clinic Hospital Design table for additional information Responsible Party: Dr. Patricia Sulak, Scott and White Hospital ClinicalTrials.gov Identifier: - Attention! // Access to the file page - // does not want the trackers to target users to obsolete versions of the study // this should be opaque to trackers who do not run javascript var yyy=new Array( ['translated','a clas','s='tr-study','-link c', 't-now','rap' t','itle="Hist','orical vers','ions of '], ['Estudio ','NCT00475553',' in Clin','icalTrials.','go'], ['v Ar','chive',' Sit','e" ','onc','lick=,'openN','ewWin', 'dow(','''/ct2/', 'history/'], ['NCT00475553', ''); return', 'false;', ['href','="/ct2/','history/','NCT00475553"', 'Council'] ['Histo','ry of C','hange','s']); var i=0; var j=0; var xxx=''; for (i=0; I selectnyyyy.length; i++) (j=0; j)yy[i].length; j++) xxx+=yyy[i][j]; document.write(xx) - No. Other Study ID Numbers: 50403 First publication: 21 May 2007 Last update: 18 December 2009 Last Verified: December 2009 stains PMS nuvaring birth control pelvic pain headache mood changes Continuous use MeSH Design Table for MeSH Terms Metrorrhagia Hemorrhagia Pathological processes Uterina Hemorragia Uterine diseases Estradiol 3-benzoate Estradiol 17 beta-cypionate Etonogestrel Desogestrel Estradiol Polyestradiol phosphate Ethinyl Estradiol Estrogens Hormones Hormones, Substitutes of Hormones and Antagonists of Hormones physiological effects of drugs Contraceptive agents Reproductive Control Agents Contraceptives, Female Contraceptives, Orals, Synthetics Contraceptives, Orals Progestins
Vaginal hemorrhagia while Nuvaring is still inserted? Medically reviewed by . Last updated on October 22, 2020. I've been at the Nuvaring for about 7 months, and I haven't had any problems. The week she was scheduled to take out the ring, the week before she started bleeding. The ring was still in, and I don't understand why I started bleeding. It wasn't heavy, but stable. I pulled the ring on Thursday instead of Friday. Now it's Monday afternoon, and I'm still bleeding, and going through some small clots. Is this happening or should I call my doctor's office? I need some information on this. Official AnswerDrugs.com Irregular bleeding may occur as a side effect while you are in NuvaRing. But if this is a change in your regular bleeding pattern, and if you are worried about it, please consult your doctor about this. For more information on the side effects of Nuvaring: Related Medical Issues Drug InformationRelated Support GroupsDrugs.com Mobile Apps The easiest way to find drug information, identify pills, check interactions, and set up your own personal drug records. Available for Android and iOS devices. SupportAboutTerms " Privacy to Drug.com newsletters for latest drug news, new drug approvals, alerts and updates. Drug.com provides accurate and independent information about more than 24,000 prescription drugs, free-sale medicines and natural products. This material is provided only for educational purposes and is not intended for medical advice, diagnosis or treatment. Data sources include IBM Watson Micromedex (updated 3 Mar 2021), Cerner MultumTM (updated 1 Mar 2021), ASHP (updated 3 Mar 2021) and others. Ad options

Possible Risks & Side Effects of NuvaRing® (etonogestrel/ethinyl estradiol vaginal ring)
Patient Information NuvaRing® (NEW-vah-ring) (etonogestrel/ethinyl estradiol vaginal ring) Hormonal birth control methods help:max_bytes(150000):strip_icc()/i-forgot-to-take-out-my-nuvaring-3869072-Final-15e924a1f5c645a69bf4e3ec440c5bb5.png)
What to Do If You Forget to Take Out Your NuvaRing
What Is Withdrawal Bleeding? On Birth Control, After Stopping, More
NuvaRing: 14 Things You Should Know Before Using the Vaginal Ring | SELF
Patient Information NuvaRing® (NEW-vah-ring) (etonogestrel/ethinyl estradiol vaginal ring) Hormonal birth control methods help
NuvaRing: 14 Things You Should Know Before Using the Vaginal Ring | SELF
Period vs breakthrough bleeding vs implantation bleeding - Page 1 | BabyCenter
NuvaRing (Etonogestrel, Ethinyl Estradiol Vaginal Ring): Uses, Dosage, Side Effects, Interactions, Warning
Patient Information NuvaRing® (NEW-vah-ring) (etonogestrel/ethinyl estradiol vaginal ring) Hormonal birth control methods help
NuvaRing: 14 Things You Should Know Before Using the Vaginal Ring | SELF
Package leaflet: Information for the user NuvaRing® 0.120 mg/0.015 mg per 24 hours, vaginal delivery system Etonogestrel/Ethiny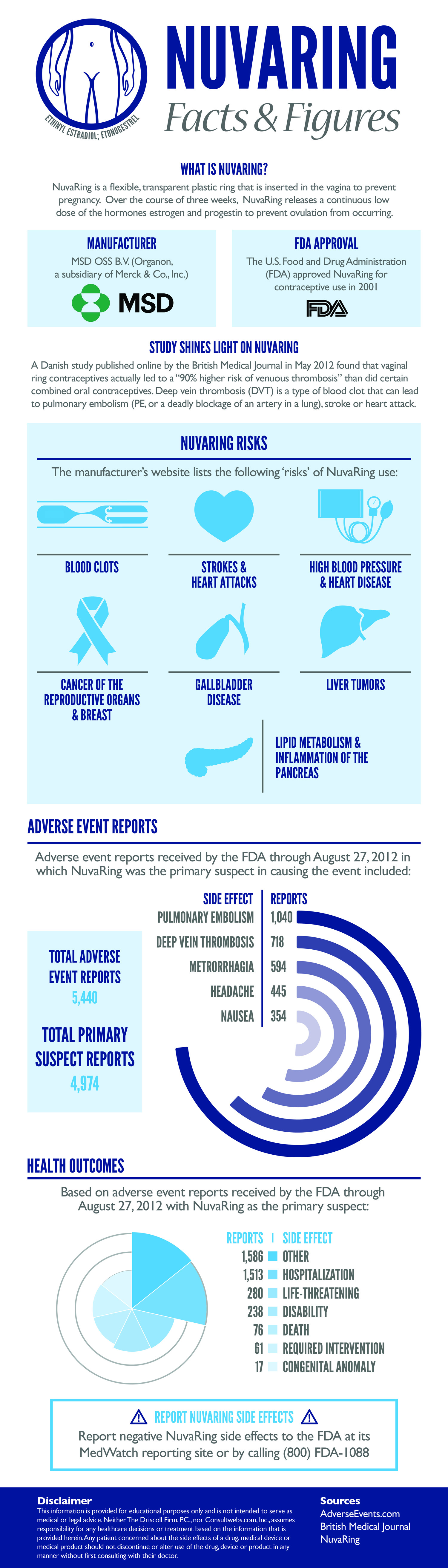
Page 2 for Using The NuvaRing As Birth Control - Stay at Home Mum
Vaginal Hormonal Ring (NuvaRing®, Annovera™ ) | Center for Young Women's Health
NuvaRing: 14 Things You Should Know Before Using the Vaginal Ring | SELF
Patient Information NuvaRing® (NEW-vah-ring) (etonogestrel/ethinyl estradiol vaginal ring) Hormonal birth control methods help
Merck's NuvaRing Settlement Doesn't Stop Some Women | Time
NuvaRing: 14 Things You Should Know Before Using the Vaginal Ring | SELF
Vaginal Hormonal Ring (NuvaRing®, Annovera™ ) | Center for Young Women's Health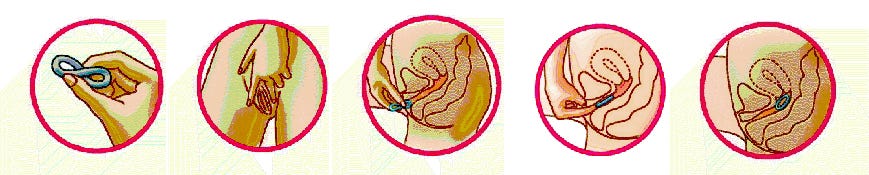
Why You Shouldn't Worry About NuvaRing | by Erin Biba | LadyBits on Medium | Medium/i-forgot-to-take-out-my-nuvaring-3869072-Final-15e924a1f5c645a69bf4e3ec440c5bb5.png)
What to Do If You Forget to Take Out Your NuvaRing
Acceptability, tolerability, and satisfaction of a contraceptive vaginal ring (the NuvaRing) among Thai women in: Asian Biomedicine Volume 10 Issue 3 (2017)
Patient Information NuvaRing® (NEW-vah-ring) (etonogestrel/ethinyl estradiol vaginal ring) Hormonal birth control methods help
Implantation Bleeding: What It Is and What to Look For (Photos!)
NuvaRing - NPS MedicineWise
Birth Control And Blood Clots: Women Still Weighing The Risks : Shots - Health News : NPR
NuvaRing - FDA prescribing information, side effects and uses
Birth Control And Blood Clots: Women Still Weighing The Risks : Shots - Health News : NPR
Managing implant users' bleeding and spotting - Contraceptive Technology
Package leaflet: Information for the user NuvaRing® 0.120 mg/0.015 mg per 24 hours, vaginal delivery system Etonogestrel/Ethiny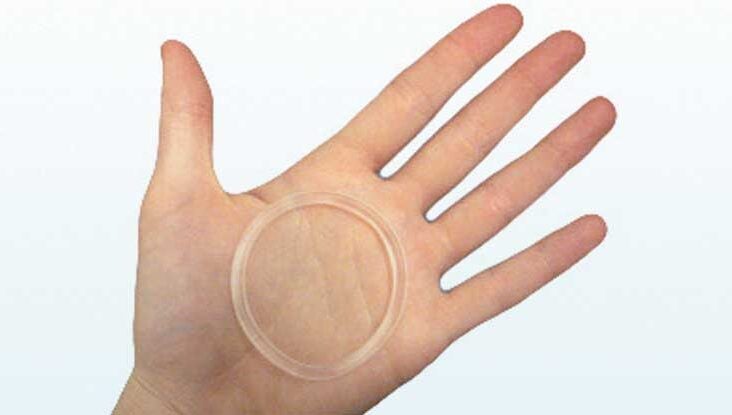
Questions About NuvaRing: Use, Side Effects, and More
NuvaRing 101: Birth control ring benefits and side effects
NuvaRing: 14 Things You Should Know Before Using the Vaginal Ring | SELF
What is Withdrawal Bleeding? | Bleeding on Birth Control | Natural Cycles
Patient Information NuvaRing® (NEW-vah-ring) (etonogestrel/ethinyl estradiol vaginal ring) Hormonal birth control methods help
Combined results from two studies examining the incidences (expressed... | Download Scientific Diagram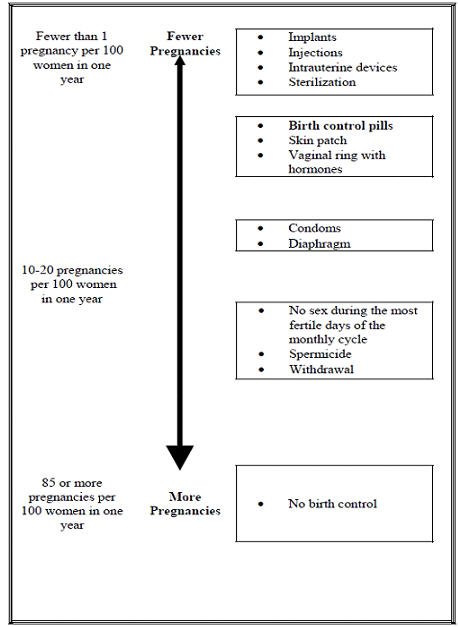
NuvaRing - FDA prescribing information, side effects and uses
NuvaRing (MSD) Drug / Medicine Information
Reference ID: 3992944
NuvaRing - NPS MedicineWise

:max_bytes(150000):strip_icc()/i-forgot-to-take-out-my-nuvaring-3869072-Final-15e924a1f5c645a69bf4e3ec440c5bb5.png)












/i-forgot-to-take-out-my-nuvaring-3869072-Final-15e924a1f5c645a69bf4e3ec440c5bb5.png)














Posting Komentar untuk "bleeding with nuvaring still in"